When lutetium-177 therapy got its first FDA approval, we awaited a brand name — most likely three syllables, middle one stressed, like Zytiga, Xofigo, Xtandi, Jevtana, Lynparza, Erleada, Nubeqa 1 — and we got, sure enough, Pluvicto.
Unexpectedly, though, we also got a new generic name. Lu-177–PSMA-617 became lutetium Lu 177 vipivotide tetraxetan.
It makes you wonder how generic names are picked and who decides. The names have no resemblance to the chemical names, in case you thought they might.
And setting that aside, what in fact is, or was, PSMA-617? How is it different from PSMA-I&T? What's the J617 in Lu-177–J617?
We'll start with the second set of questions because they influence the answers to the first.
The ligand formerly known as PSMA-617
As you can imagine, attaching lutetium, a metal, to PSMA, a biological molecule, requires a chemical shim.
The solution has been a single three-part molecule — one part to bind the lutetium, one to bind the PSMA, and one to tie those ends together.
The lutetium end is a chelator, which attaches a metal to an organic molecule. The PSMA end is a pharmacophore or binding motif, which chemically adheres to a precise point on the PSMA molecule. The linker that joins them is just called a linker.
A substance that binds to a site on a target molecule, as this does, is known as a ligand.
Alternatives in picking the three components lead to a proliferation of PSMA ligands.
Moreover, a chelator might provide a choice of metal, such as Ac-277–PSMA-617 for therapy and Ga-68–PSMA-617 for PET.
PSMA I&T has the PSMA-617 binding motif, different linker and chelator. It’s usable for Imaging or Therapy (as is PSMA-617) and works with a similar gallery of radioactive metals.

It’s been argued that imaging and therapy should use the same ligand; mapping them to precisely the same tissues will show unambiguously whether treatment is working.2 Though that’s possible in PSMA-617 and PSMA-I&T it doesn’t seem to be widely exploited, and perhaps the benefit is more theoretical than practical.
Part of what drives ligand development is a desire to minimize affinity to nonprostate tissues. A 2021 study suggests individual responses are so varied that it’s hard to declare PSMA-617 or PSMA-I&T better.
Bodies and antibodies
PSMA is meant to be a meeting place for other molecules. It is a facilitator for chemical reactions — an enzyme, or organic catalyst.
Other molecules can be designed that make use of this come-hither property and bind to PSMA. A molecule preemptively occupying a site intended for other molecules is called an inhibitor — even in cases like this, where the intention is less to inhibit PSMA than to tie a radioactive tail on it.3
Chemists had to craft a molecular physique to fit the PSMA pocket. Nature, though, is prepared to do it for free with an antibody4. The pharmacophore in the PSMA-J591 ligand is an antibody; drugs with antibody ligands are called antibody-drug conjugates. The difference is, literally, huge. You’ll hear drugs with lab-designed ligands called “small molecule” in part because antibodies, like most biological molecules, are big. PSMA-J591 is 100 times the size of PSMA-617.
Because they’re bigger, antibody-drug conjugates take longer to reach a target; they remain in the body longer; and they aren't able to reach all the places small molecules go.
The first of these makes them awkward for PET scans; instead of getting your scan an hour after getting the tracer, you return days later.
The second may cause them to overstay their welcome and accumulate in liver, spleen, and elsewhere, and to damage bone marrow. The point is made emphatically (and in two languages) here:

The third property might help keep the radionuclide away from PSMA-expressing non-targets. Size restricts antibodies to the large blood vessels characteristic of tumors. On the other hand, it’s also argued (as in the slide) that the size limits tumor penetration.
Hello, Vippy!
And now, vipivotide tetraxetan. As a generic-drug name, it was born with most of its syllables already in place.
PSMA-617 had to be a -votide and an -xetan:
-votide designates a PSMA peptide (the pharmacopore part).
-xetan is for a chelating agent. Tetraxetan was already there for the PSMA-617 chelating agent.
Endocyte Ltd. (now part of Novartis) then got more-or-less free choice over the remaining two syllables, and vipivotide tetraxetan got voted in as a nonproprietary drug name, accompanied by an authorized pronunciation:5
Who’s in charge here?
The United States Adopted Names Council, which maintains a slowly growing list of about 650 partial names based on drug types. Because of this standardization, you can tell, based only on its generic name, how sipuleucel-T works.
To these USAN building blocks, a drug maker gets to add a few syllables — which must sound distinctive enough to avoid pharmacist mixups, use only letters available in foreign alphabets (no H, J, K, W), suggest nothing excessively good or bad (no new, mal, mor), and so on. The letters also can't suggest the brand name.
Brand names, in contrast, are free to roam the alphabet and take their pick of syllables. They just need FDA approval.
In poetry, that middle-syllable stress is called an amphibrach. You find it in limericks: “There was a | young girl from | Nantucket.” Consumer meds seem to favor the first syllable — Nexium, Bufferin, Lotrimin, Klonopin, Alka-Seltzer, Sudafed — though we do have Eligard, Casodex, Firmagon, Taxotere. Two-syllable names seem to have gone the way of Darvon and Quaalude (with 80's Lupron hanging in). Provenge — two syllables, stress on first, 2010 — breaks all the rules.
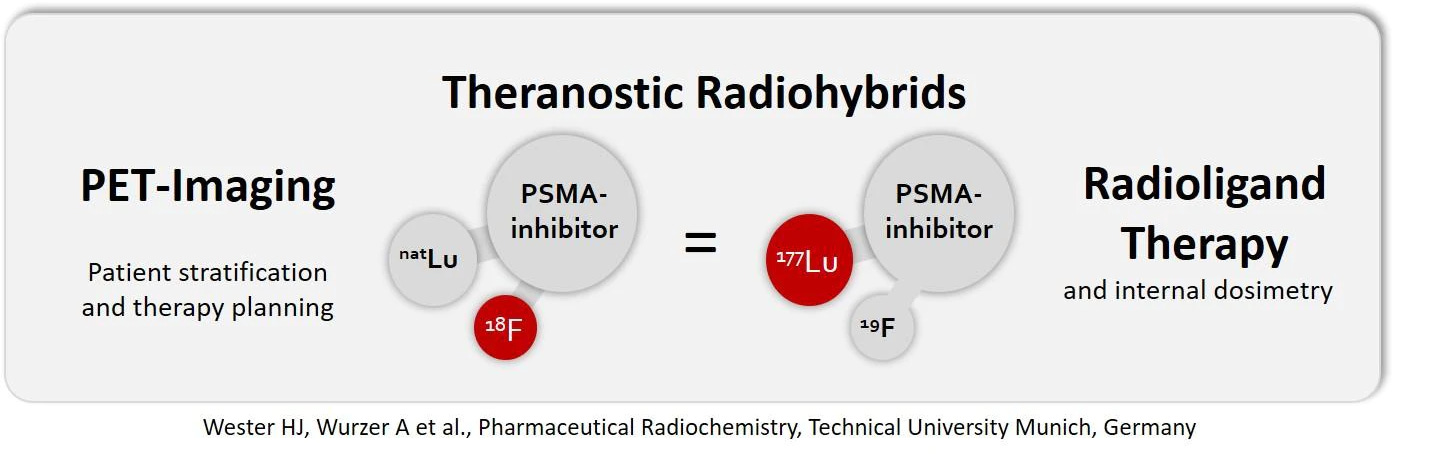
A radiohybrid theranostic architecture has been invented that makes the change even smaller. Swapping gallium and lutetium is a change on a chemical level, even if nothing else is altered. A radiohybrid always has the same two elements whether it’s used in therapy or a scan; one of the two is chosen to be a radioactive version (radioisotope) depending on the application. Scans use a radioactive fluorine and nonradioactive lutetium (F-18, Lu-175), and therapy uses a radioactive lutetium and nonradioactive fluorine (F-19, Lu-177). Chemically, the scan and therapeutic versions could not be closer. This is the 177Lu-rhPSMA-10.1 that Blue Earth has begun trials on.
PSMA does play a role in prostate cancer progression, so simply plugging it without a radioactive payload would have a minor benefit.
We won’t cover here the fascinating story of how an antibody can be made to order.
An official pronunciation helps ensure that doctors instructing pharmacists will be correctly heard. So you don’t have to be the guy who says de-GAR-a-lix thinking the pronunciation of degarelix is anyone’s guess. You can look it up: